
However, there was an untimely delay in the publication of his most elaborate periodic table, and, perhaps more importantly, Meyer-unlike Mendeleev-hesitated to make predictions about unknown elements.

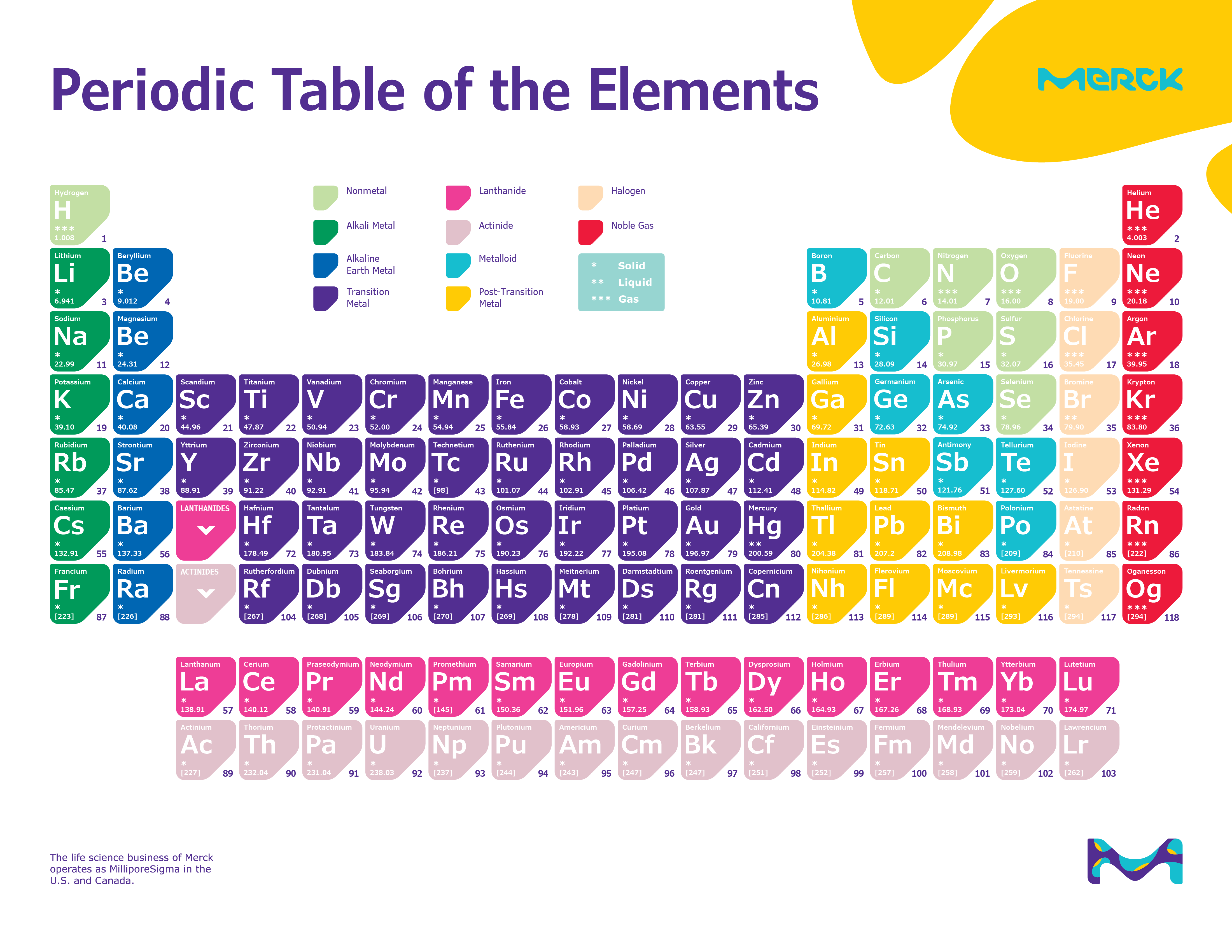
The closest precursor to Mendeleev’s table in both chronological and philosophical terms was developed by Julius Lothar Meyer, a German chemist, in 1864. By the 1860s a number of scientists had moved beyond the triad concept to produce some very respectable periodic systems. The elements in these groupings displayed an important numerical relationship to each other: the equivalent weight (an early substitute for atomic weight) of the middle element had the approximate mean of the values of the two flanking elements. The observation that certain types of elements prefer to combine with certain other types prompted early chemists to classify the elements in tables of chemical affinities. Mendeleev was hardly the first to arrive at a periodic system. It marks the 150th anniversary of the publication by the Russian chemist Dmitry Mendeleev (1834–1907) of his Periodic Table and celebrates the significance and impact of this outstandingly successful chart of the atomic building blocks of matter. The year of 2019 was designated by the United Nations as the International Year of the Periodic Table of Chemical Elements. This periodic table chart lists elements by name in alphabetical order including the element symbol and atomic number for quick and simple reference.

PERIODIC TABLE CHEMISTRY PDF FREE
30 Free Printable Periodic Tables by Science Notes (PDF, PNG).Have fun!ĥ6 Free Periodic Tables – Printable for All Your Chemistry Adventures
PERIODIC TABLE CHEMISTRY PDF DOWNLOAD
Feel free to download and print for your own personal use in whatever project that you’re currently in. The table can be used for predicting the properties of elements, including those not yet discovered.īelow you’ll find 56 printable periodic tables in various forms, designs and layouts. To summarise, the periodic table is useful as it is arranged in one easy-to-use guide to provide a lot of details about elements and how they relate to each other.
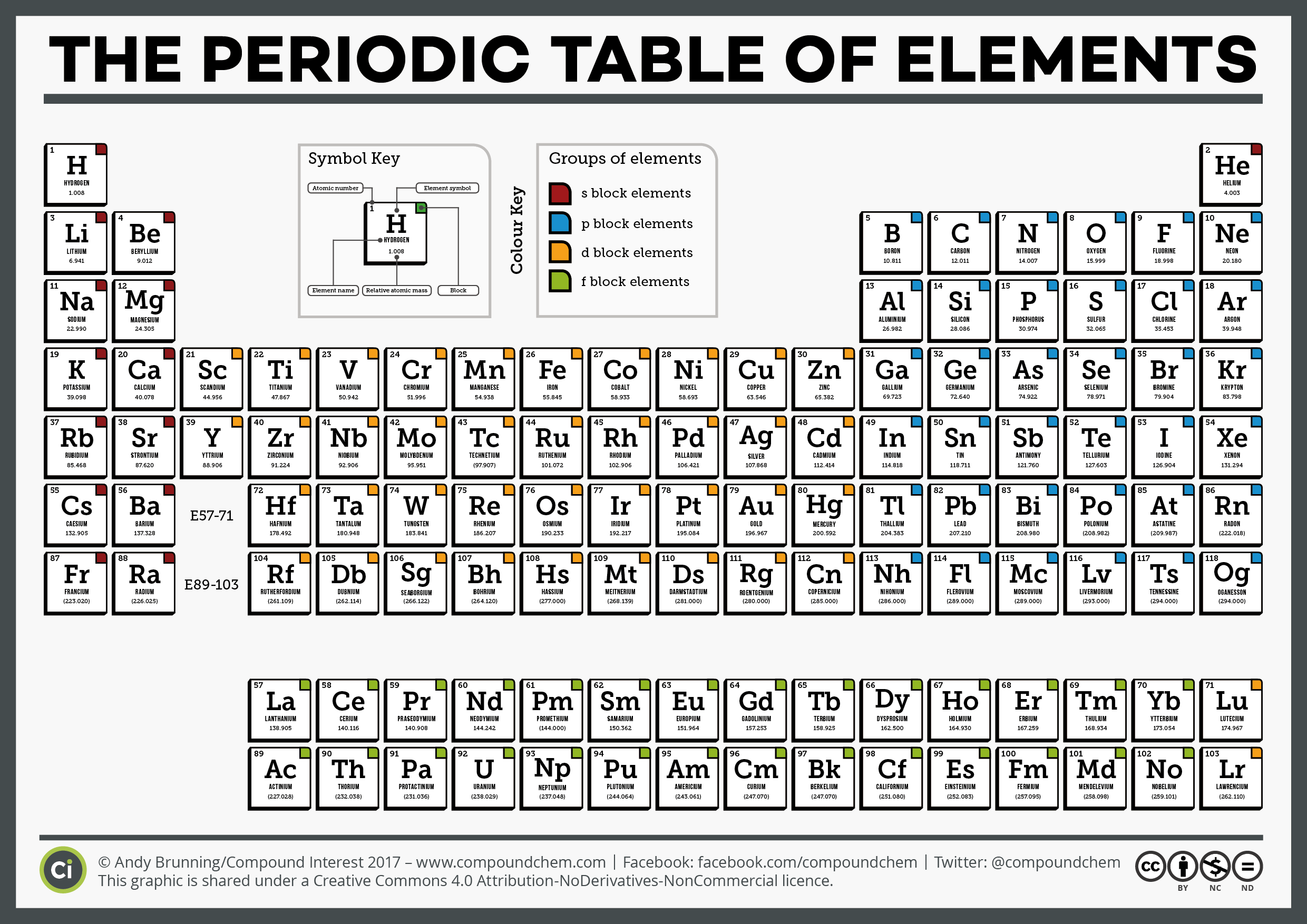
Order correlates usually with increased atomic mass. As the atomic number increases, the elements are distributed from left to right and top to bottom. The periodic table of elements arranges in a detailed collection of all known chemical elements. The atomic number of the element is the number of protons in the nucleus of the element. The periodic table is a tabular array of chemical elements organized by atomic number, from the lowest atomic number element, hydrogen, to the highest atomic number element, oganesson.


 0 kommentar(er)
0 kommentar(er)
